Say goodbye to embrittlement and high-temperature failure! A Chinese research team has overcome a critical bottleneck in epoxy resin curing!
This paper reviews recent research on thermal curing, microwave curing, and photocuring technologies of epoxy resins both domestically and internationally. In the field of thermal curing technology, it focuses on several functional curing agents with high heat resistance, flame retardancy, and toughness, as well as several novel modified amine curing agents. A preliminary analysis and discussion of microwave curing systems for epoxy resins is presented through comparison with thermal curing. In the field of photocuring technology, the paper mainly summarizes the progress of cationic UV curing systems and free radical-anionic hybrid photocuring systems for epoxy resins.
- Introduction
Epoxy resins are commonly used as adhesives, casting materials, fiberglass, and coatings. Their excellent properties, such as adhesion, electrical insulation, chemical resistance, high strength, high heat resistance, and the development of various new products, have led to their increasingly widespread application in mechanical engineering, aerospace, electrical engineering, chemical industry, and other fields. To further improve the performance of cured epoxy resins, modifications can be made to the resin matrix by altering the resin structure or synthesizing new resins; alternatively, additives can be used to achieve high-performance characteristics. Research and utilization of novel curing technologies are also becoming a major approach.
- Thermosetting Systems
With the gradual deepening of research on epoxy resin matrices and their modification, significant progress has been made in epoxy resin curing technology. Various new curing agents with special properties have become a research hotspot in the application of general-purpose epoxy resins both domestically and internationally in recent years.
2.1 Flame-Retardant and High Heat-Resistant Curing Agents: Improving the flame retardancy of epoxy resins can be achieved by physically incorporating flame-retardant additives. However, these additives are prone to leaching during use, and the process is complex. Conversely, incorporating flame-retardant substances into the polymer backbone structure through chemical bonds not only achieves permanent adhesion of the flame-retardant groups to the resin but also has little impact on its physical and mechanical properties. Compared with traditional bromine-containing flame retardants, organophosphorus compounds exhibit high flame-retardant performance while producing fewer toxic substances. Shieh et al. synthesized the phosphorus-containing compound ODC by reacting o-phenylphenol with phosphoryl chloride, and then synthesized a new phosphorus-containing flame-retardant curing agent OD-PN using ODC and linear phenolic resin (PN). The reaction equation is as follows:
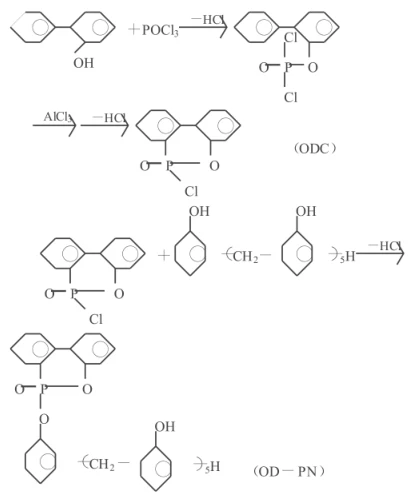
Epoxy resins cured with this curing agent, due to their regular structure containing ODC and phosphorus-containing side groups, exhibit higher flame retardancy, higher glass transition temperature, and thermal stability compared to epoxy resins cured with non-cyclic phosphorus-containing curing agents or bromine-containing curing agents. Cresol formaldehyde epoxy resin (CNE) cured with this curing agent can achieve the UL94-VO standard with a phosphorus content of only 1.21% (compared to 6% bromine content required for bromine-containing curing agents), and does not release toxic fumes; at a phosphorus content of 1.72%, the Tg is 178℃, and only 10% weight loss occurs at 401℃ in air, with an LOI value (the minimum percentage of oxygen in an oxygen-nitrogen mixture that can support the combustion of a substance) of 35. The F-series epoxy resin curing agent successfully developed by Zhang Duotai is a modified phenolic resin, and some of it has been commercialized. This curing agent allows general-purpose epoxy resins to withstand high temperatures of 300-400℃, possessing clean and safe high flame retardancy and outstanding ablation resistance. Even if the cured material decomposes at high temperatures, the residual carbon layer still retains some of the properties of the raw materials. The F curing agent itself is non-polluting, can be stored for a long time, and is easy to use; it can be used in powder or solution form; it can be cured at high or medium temperatures, and also has a self-coloring function, making it a multi-purpose and highly efficient curing agent. The thermogravimetric analysis (TG) curves and differential thermogravimetric curves (DTG) of E-51 epoxy resin and multi-functional epoxy resin AFG-90 cured with F-series curing agents F-52B and F-52A, respectively, are shown in Figure 1.
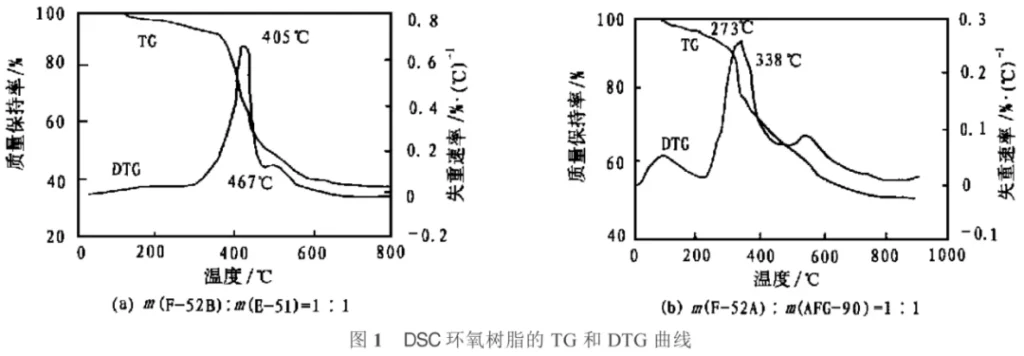
As shown in Figure 1, the decomposition temperature of the cured E-51 epoxy resin is approximately 380℃, with the maximum weight loss rate occurring at 405℃. After 467℃, the weight loss slows down, and the mass retention rate at 800℃ is still 38.17%. The decomposition temperature of the cured multifunctional epoxy resin AFG-90 is lower than that of E-51 epoxy resin, but its mass retention rate above 800℃ can reach over 50%. In contrast, typical heat-resistant phenolic resins have a mass retention rate close to zero at 600-700℃.
2.2
Toughening Curing Agents: Generally, cured epoxy resins are quite brittle, and the brittleness of epoxy resins with a highly cross-linked, rigid network structure is even more pronounced at low temperatures. Non-reactive toughening agents will slowly volatilize over time and under the influence of light and heat, causing the resin to age and become brittle; reactive toughening agents generally have larger molecules and their reaction with the resin matrix will alter some of its properties. Therefore, the synthesis of toughening curing agents is of considerable significance. Li Qingxiu et al. successfully synthesized a toughening curing agent by reacting an anhydride with a series of flexible chain oligomers of different relative molecular weights. Tests showed that this curing agent has good compatibility with epoxy resin and can be chemically bonded to the epoxy resin network after the curing reaction; at the same curing agent dosage ratio, when the relative molecular weight of the flexible molecules in the curing agent reaches a certain value, a synergistic effect will occur in the mechanical properties of the epoxy resin, that is, while lowering the glass transition temperature (Tg), the impact strength σi, tensile strength σt, and flexural strength σf simultaneously show peak values. The relationship between the impact strength and tensile strength of the cured product and the curing agent content is shown in Figures 2 and 3.
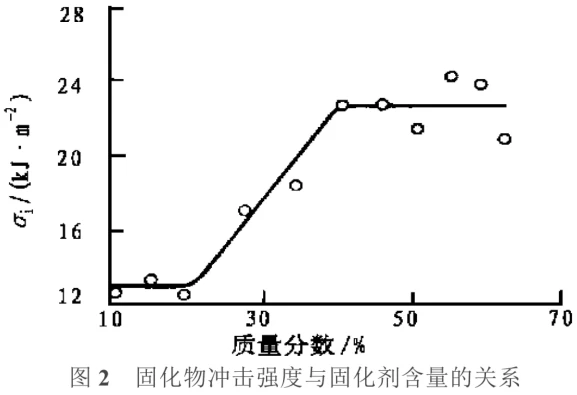
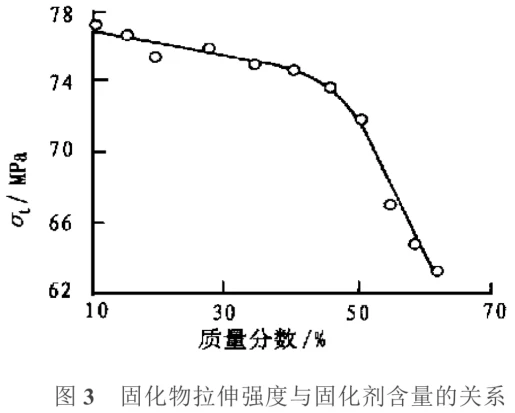
As shown in Figure 2, the impact strength of the epoxy resin modified with the curing agent is significantly higher than that of the unmodified resin. When the curing agent content is less than 40%, the impact strength increases with increasing curing agent content; when the curing agent content is greater than 40%, the impact strength remains essentially unchanged. This indicates that the curing agent content only needs to reach a certain value to effectively improve the brittleness of the epoxy resin. As shown in Figure 3, the tensile strength gradually decreases with increasing curing agent content, and the tensile strength decreases significantly after the curing agent content reaches 50%. This is because the crosslinking density of the epoxy resin decreases to some extent with the increase of flexible chains.
2.3 Modified Amine Curing Agents
To overcome the brittleness, poor impact resistance, inadequate weather resistance, and toxicity of amine curing agents, further modification of amine curing agents is necessary to obtain non-toxic or low-toxicity amine curing agents that can cure at room temperature. Currently used amine curing agents, such as m-phenylenediamine, 4,4′-diaminodiphenyl sulfone, and 4,4′-diaminodiphenylmethane, have limited applications due to their toxicity, low solubility, and high exothermic properties. Liu Pei et al. synthesized 4,4′-bis(3-aminophenoxy)diphenyl sulfone (m-BAPS), a novel aromatic diamine curing agent that improves the structural properties of epoxy systems, increases toughness and moisture and heat resistance, and lowers the curing temperature. This curing agent was also refined using the hydrochloride salt method. Addressing the inconvenience of using aqueous formaldehyde solutions in previous modified amine curing agents, Bai Liying used paraformaldehyde instead of aqueous formaldehyde solution, reacting paraformaldehyde with urea and phenol in a solvent to produce a non-volatile urea-phenol-formaldehyde condensate. This condensate was then reacted with ethylenediamine to produce a novel epoxy resin curing agent, UPFA. This process avoids the use of aqueous formaldehyde solution, eliminating the introduction of water into the system, simplifying product processing, and reducing ethylenediamine loss. It also lowers costs, allows for milder reaction conditions, and facilitates industrialization and commercialization of the product. Dicyandiamide is one of the most commonly used latent curing agents, but it has a high curing temperature and poor solubility in epoxy resins, which is unfavorable for wet molding. Modification with aniline-formaldehyde improves its properties; the modified dicyandiamide is soluble in a mixed solvent of acetone and alcohol, and under the action of acrylic acid-passivated imidazole, it can cure the resin at a moderate temperature of 125°C. The resulting cured product exhibits good room temperature and wet heat performance. Traditional fatty amine curing agents have been gradually phased out due to their high toxicity. Currently, T31 modified amine curing agents are commonly used, but their cured products are relatively brittle and have low impact strength. Yuan Jinsong et al. synthesized a new Tcs curing agent, which exhibits comparable curing performance to T31, but shows a significant improvement in impact strength.
Microwave curing system
In the past decade, microwaves have attracted considerable attention as an energy source in materials preparation processes. Compared with traditional heating methods, microwaves offer advantages such as uniform heat transfer, high heating efficiency, and ease of control. Numerous studies have reported on organic reaction systems controlled by microwaves, but there is still no consensus on whether a “microwave effect,” i.e., a non-thermal effect, exists during these chemical reactions. Microwaves can also be used in polymer reaction systems, and extensive research has been conducted on the microwave curing of epoxy resins. Wei et al. compared the microwave curing reactions of two systems, bisphenol A epoxy resin (DGEBA)/m-phenylenediamine (mPDA) and bisphenol A epoxy resin (DGEBA)/diaminodiphenyl sulfone (DDS), with traditional thermal curing reactions. Microwave curing showed a higher reaction rate, and the glass transition temperature (Tg) of the cured product increased significantly. Marand and Gray-beal studied the microwave curing process of DGEBA/DDS and found that microwaves increased the reaction rate in the early stages of curing, but decreased the reaction rate in the later stages, ultimately leading to a decrease in the degree of curing. Mijoric and Wijaya also studied the same system, but reported varying degrees of decrease in both the reaction rate and the Tg of the cured product. Most of these studies directly compared the reaction process and product properties of microwave curing and thermal curing, but in reality, the two processes differ significantly. In traditional heating, energy is transferred gradually through a temperature gradient from the surface to the interior of the material, while microwaves can uniformly heat the entire system; traditional heating allows for direct temperature measurement using thermocouples, enabling timely control of temperature and pressure, while in microwave systems, to avoid the thermocouple disturbing the electromagnetic field, only indirect temperature measurement using a fluorescence calorimeter is possible, resulting in a certain lag in temperature and pressure control; in addition, the spatial shape of the microwave container used has a certain influence on the electromagnetic field distribution, and ultimately affects the reaction process itself. These differences may be the reason for the inconsistencies in the reported results. Baofu and Martin constructed pulsed power and continuous power microwave systems and used these two systems to conduct isothermal curing reactions of bisphenol A epoxy resin (DGEBA) with three curing agents: diaminodiphenylmethane (DDM), m-phenylenediamine (mPDA), and diaminodiphenyl sulfone (DDS) at different temperatures. The temperature fluctuations in these two systems were controlled within 1°C and 0.5°C, respectively. The larger temperature fluctuation in the pulsed power system was due to changes in the dielectric properties of the reactants caused by power variations, resulting in a certain lag in the reactants returning to their response state before the power was switched on and off. The reaction process was monitored by two parameters: incident power and reflected power. The degree of curing was determined during the reaction using Fourier transform infrared spectroscopy. Analysis of the curing degree-time curves showed that microwave curing exhibits distinct thermal curing characteristics, including three reaction stages: initial, self-accelerating, and physical precipitation. The higher the curing temperature, the shorter the first two stages, and the higher the degree of curing of the product. If microwaves primarily increase the reaction rate through thermal effects, when the power is zero, the decrease in reaction rate should be limited because the entire system is controlled to maintain a constant temperature; if the reaction rate is mainly determined by non-thermal effects, when the power is zero, the microwave effect completely disappears, and the reaction rate must decrease significantly. That is, the reaction rate of the continuous power microwave system should be significantly higher than that of the pulsed power microwave system, which is confirmed by the experimental results of this study. Due to the influence of the pulse magnitude and pulse cycle in the pulsed power system, further investigation is needed to obtain definitive conclusions.
Photopolymerization system
Ultraviolet (UV) curing of epoxy resins refers to the process where, under UV light, photosensitive substances in the system undergo photochemical reactions to produce active particles or groups, thereby initiating the cross-linking polymerization of the active resin in the system. This technology does not require the use of organic solvents, resulting in minimal environmental pollution. It also offers advantages such as fast curing speed, energy savings, good product performance, and suitability for high-speed automated production lines and coating heat-sensitive substrates. Currently, commonly used UV curing systems are classified into free-radical photocuring systems and cationic photocuring systems based on the type of initiator system. Free-radical UV curing systems have fast reaction speeds and easily adjustable properties, but they are sensitive to oxygen, exhibit high photocuring shrinkage, poor adhesion, and are difficult to completely cure three-dimensional parts. Therefore, cationic UV curing has become a popular research and development area in recent years. Novel hybrid photocuring systems that can undergo both photo-free radical polymerization and photo-cationic polymerization have also become an active research and development field. Furthermore, to further expand the application range of photocuring and improve the performance of photocured products, dual-curing systems that combine photocuring with other curing methods are also continuously being researched and explored.
4.1 Cationic UV-Curing Systems
Cationic photocuring refers to the process where a cationic photoinitiator, under UV irradiation, generates a protonic acid or Lewis acid, forming a positively charged active center that initiates cationic ring-opening polymerization. Compared to photoinitiated free-radical polymerization, cationic curing has the following characteristics: a. It is applicable to a wide variety of monomers, including not only monomers and prepolymers containing unsaturated double bonds but also various monomers and prepolymers with ring strain, such as acetals, cyclic ethers, epoxides, β-lactones, sulfides, and silicones. b. It is not inhibited by oxygen, allowing for rapid and complete polymerization in an air atmosphere, which is beneficial for production and practical applications. c. It exhibits post-curing effects, which can shorten irradiation time, improve production efficiency, and enhance product quality in practical applications. The selection of the initiator is the most important issue in cationic curing systems, as it directly determines the curing and crosslinking speed. The earliest developed cationic initiators were diazonium salts:
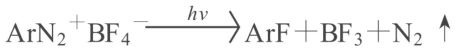
The generated BF3 is a Lewis acid, which can directly initiate cationic polymerization, or react with H2O or other compounds to generate protons (H+), which then initiate polymerization. Besides BF4-, the diazonium salt anion can also be PF6-, AsF6-, and SbF6-, etc. The disadvantage of diazonium salt initiators is that N₂ is released during photolysis, making them unstable and unsuitable for long-term storage. Representatives of the new generation of anionic photoinitiators are iodonium salts and sulfonium salts: Ar₃S+X¯, Ar₂I+X¯, where X¯ can be SbF6-, AsF6-, PF6-, or BF4-. The curing speed of onium salt initiators depends on the activity of the anion. Studies have shown that the activity order is SbF6¯ > AsF6¯ > PF6¯ > BF4¯, and Stuart et al. showed that [B(PhF5)4]¯ has even stronger activity than SbF6¯. The maximum absorption spectra of sulfonium and iodonium salts are in the far ultraviolet region, with no absorption in the ultraviolet region. The spectral characteristics of onium salts can be improved by increasing the conjugation degree of the onium salt, shifting its maximum absorption towards longer wavelengths, or by forming a composite initiator with a sensitizer. Commonly used sensitizers are some free radical photoinitiators, such as pyrene, anthracene, and thiazine, or thioxanthone and xanthone, but the latter two can only be used with iodonium salts. Peng Changzheng et al. synthesized various triaryl sulfonium hexafluoroantimonates and studied the factors affecting the cationic photocuring speed of epoxy polymethylsiloxane (EPS) and bisphenol A epoxy resin E-44 using these as photoinitiators. The results showed that the structure and concentration of the photoinitiator, and sensitizers such as anthracene, phenol, and thiazine, all have varying degrees of influence on the photocuring speed. This method can yield photocuring compositions with fast curing speeds and good mechanical properties, exhibiting significant post-curing characteristics due to living polymerization. These materials are expected to find applications in high-tech fields such as photocuring coatings, adhesives, sealants, electrical insulation materials, and electronic component encapsulants. The general structural formula of this initiator is:
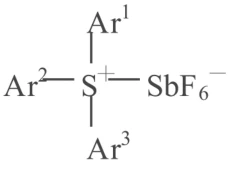
In the formula, Ar’ and Ar³ represent phenyl groups, and Ar² represents benzene, toluene, or tert-butylbenzene. The curing mechanism is as follows: the excited state of the triaryl sulfonium hexafluoroantimonate, which absorbs ultraviolet light energy of a specific wavelength, further decomposes to generate free hexafluoroantimonic acid HSbF6. According to Crivello’s photolysis theory, the reaction is as follows:
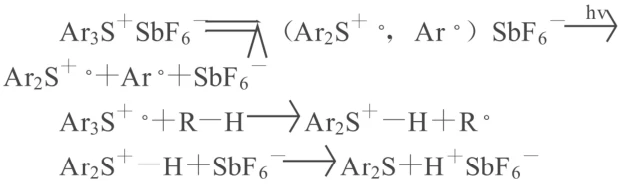
In the formula, R-H represents a compound containing active hydrogen. The H+SbF6¯ generated by photolysis is an active Brønsted acid, which readily dissociates into H+, thus initiating the cationic ring-opening polymerization of the multifunctional epoxy prepolymer and ultimately crosslinking into a three-dimensional polymer. The curing process can be completed simultaneously through bond growth reactions of both oxonium and carbonium ions as active centers. The disadvantage of onium salt cationic initiators is that they contain the highly toxic metal ion SbF6¯, have poor solubility in polysiloxanes, and have low thermal stability. Replacing antimony with boron can reduce its toxicity while maintaining high activity. In addition, the currently proposed three-component systems of ketone-amine-onium salt or ketone-amine-bromide can overcome the termination reaction of active particles and improve the initiation activity. 4.2 Free Radical-Cationic Hybrid Photocuring System Addressing the respective characteristics of free radical photocuring and cationic photocuring, the free radical-cationic hybrid photocuring system can combine the advantages of both, thus broadening the application range of photocuring systems. In the application of epoxy resins, acrylate free radical photocuring systems are often combined with epoxide cationic photocuring systems to form a hybrid system, which exhibits excellent synergistic effects in photoinitiation, complementary volume changes, and performance adjustment. In the acrylate-epoxy hybrid photocuring system, the addition of epoxides can balance the volume change of acrylates, reduce the volume shrinkage rate of the curing system, thereby reducing internal stress and enhancing adhesion. However, it will reduce the curing speed of the system. Increasing the acrylate content can increase the curing speed and solvent resistance of the system, but it increases the shrinkage rate and reduces the adhesion to the substrate. Therefore, according to the different requirements for the performance of the cured product, different hybrid systems can be obtained by changing the acrylate/epoxide ratio to meet different application needs. For example, a hybrid photocurable system formulated with the aliphatic epoxy compound CY179, caprolactone triol, triaryl sulfonium salt, benzophenone, and acrylate monomers exhibits high curing speed, good solvent resistance, and low volume shrinkage. This system has yielded satisfactory results when used in stereolithography. In this system, the acrylate monomer can be replaced with an epoxy acrylate oligomer, and treating the cured product at 180°C for 3 minutes can significantly improve its performance.
4.3 Dual-Curing Systems
Because the UV curing process of epoxy resins is initiated by light, this system has limitations such as restricted curing depth and difficulty in curing colored systems and shaded areas. Therefore, dual-curing systems combining photocuring with other curing methods have been developed. The cross-linking polymerization of the system is completed through two independent stages with different reaction principles. One stage is through a photocuring reaction, and the other stage is through a dark reaction, which includes thermal curing, moisture curing, and oxidative curing reactions. Dual curing expands the application of photocuring systems to opaque media, complex-shaped substrates, ultra-thick coatings, and colored coatings. In a sense, dual polymerization systems are a broad category of hybrid polymerization systems. For example, mixing bisphenol A epoxy monoacrylate, acrylate monomer, photoinitiator Irgacure 184, and epoxy resin curing agent 3-methylimidazole, followed by UV curing and then heating at 120°C for 30 minutes, showed a significant improvement in the mechanical properties of the cured product after heat treatment. The hybrid system also exhibited good adhesion properties. This is partly due to the low curing shrinkage of the epoxide, and partly because the thermal curing eliminates the internal stress generated during free radical curing. Therefore, dual-curing systems composed of epoxy resin, acrylate oligomer, free radical photoinitiator, and epoxy resin cross-linking agent can be applied to the encapsulation of electronic devices and the curing of thick coatings. Currently, bisphenol A type epoxy resins still hold the leading position in production and sales volume, and their applications are the most widespread. Therefore, research into epoxy resin curing technology and curing agents is of great significance. Traditional thermal curing is widely used, and the technical means are relatively complete. Recent research has mainly focused on developing new curing technologies and new curing agents to achieve high-performance cured products, such as improving their toughness, heat resistance, and flame retardancy. Both microwave curing and photocuring technologies have their own advantages and characteristics. Utilizing these two methods in combination with thermal curing can help improve the curing reaction system and enhance the performance of the cured products, enabling the application of high-performance epoxy resins and epoxides in specialized fields and high-tech industries.