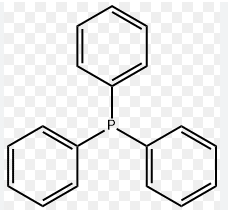
product description
Basic Info.
Triphenylphosphine Properties
| Melting point | 79-81 °C(lit.) |
|---|---|
| Boiling point | 377 °C(lit.) |
| bulk density | 500-600kg/m3 |
| Density | 1.132 |
| vapor density | 9 (vs air) |
| vapor pressure | 5 mm Hg ( 20 °C) |
| refractive index | 1.6358 |
| Flash point | 181 °C |
| storage temp. | Store below +30°C. |
| solubility | water: soluble0.00017 g/L at 22°C |
| form | Crystals, Crystalline Powder or Flakes |
| color | White |
| Specific Gravity | 1.132 |
| Odor | odorless |
| Water Solubility | Insoluble |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,9743 |
| BRN | 610776 |
| Stability | Stable. Incompatible with oxidizing agents, acids. |
| Cosmetics Ingredients Functions | NAIL CONDITIONING |
| InChI | 1S/C18H15P/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H |
| InChIKey | RIOQSEWOXXDEQQ-UHFFFAOYSA-N |
| SMILES | c1ccc(cc1)P(c2ccccc2)c3ccccc3 |
| CAS DataBase Reference | 603-35-0(CAS DataBase Reference) |
| FDA UNII | 26D26OA393 |
| NIST Chemistry Reference | Phosphine, triphenyl-(603-35-0) |
| EPA Substance Registry System | Triphenylphosphine (603-35-0) |
| UNSPSC Code | 12352128 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H317-H318-H372 |
| Precautionary statements | P260-P280-P301+P312-P302+P352-P305+P351+P338-P314 |
| target organs | Central nervous system,Peripheral nervous system |
| PPE | dust mask type N95 (US), Eyeshields, Faceshields, Gloves |
| Hazard Codes | Xn,N |
| Risk Statements | 22-43-53-50/53-48/20/22 |
| Safety Statements | 36/37-60-61-36/37/39-26 |
| RIDADR | 3077 |
| WGK Germany | 2 |
| RTECS | SZ3500000 |
| F | 9 |
| Autoignition Temperature | 425 °C |
| TSCA | TSCA listed |
| HS Code | 29310095 |
| Storage Class | 6.1C – Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 4 Oral Eye Dam. 1 Skin Sens. 1B STOT RE 1 Inhalation |
| Hazardous Substances Data | 603-35-0(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 700 mg/kg LD50 dermal Rabbit > 4000 mg/kg |
Triphenylphosphine Chemical Properties,Uses,Production
Chemical properties
Triphenylphosphine is a common organophosphorus compound with the formula P(C6H5)3 – often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless to pale yellow monoclinic crystals at room temperature. It is a colorless to pale yellow transparent oily liquid above the room temperature with skin irritation and a pungent odour. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
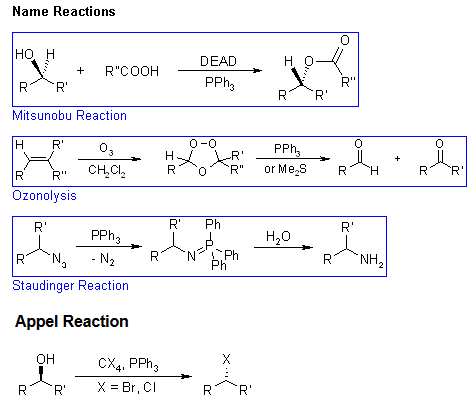
Name Reactions
- Mitsunobu reactions
The triphenylphosphine combines with DEAD to generate a phosphonium intermediate that binds to the alcohol oxygen, activating it as a leaving group. Substitution by the carboxylate, mercaptyl, or other nucleophile completes the process. - Ozonolysis reactions
Ozonolysis allows the cleavage of alkene double bonds by reaction with ozone. Depending on the work up, different products may be isolated: reductive work-up gives either alcohols or carbonyl compounds, while oxidative work-up leads to carboxylic acids or ketones. - Staudinger reactions
Triphenylphosphine reacts with the azide to generate a phosphazide, which loses N2 to form an iminophosphorane. Aqueous work up leads to the amine and the very stable phosphine oxide. - Appel reactions
The reaction of triphenylphosphine and tetrahalomethanes (CCl4, CBr4) with alcohols is a ready method to convert an alcohol to the corresponding alkyl halide under mild conditions. The yields are normally high.
This reaction is somewhat similar to the Mitsunobu Reaction, where the combination of a phosphine, a diazo compound as a coupling reagent, and a nucleophile are used to invert the stereochemistry of an alcohol or displace it.
Uses
Triphenylphosphine is first sulfonated with oleum to form the trisulfonic acid.
Triphenylphosphine can be used in Wittig synthesis. It is a standard ligand in homogeneous catalysis.
Triphenylphosphine is used in the synthesis of an organophosphorus intermediate, trimethyl phosphite in ester exchange method. And then a series of organophosphorus pesticides such as dichlorvos, monocrotophos and phosphamidon can be further obtained.
In addition, it can be used as stabilizers in the synthesis of rubber and resins, antioxidants in polyvinyl chloride, and raw material in the synthesis of alkyd resins and polyester resins.
Production methods
In this preparation method, phenol and phosphorus trichloride was used as raw materials. After esterification and vacuum distillation, the product namely triphenyl phosphite can be obtained.
3C6H5OH + PCl3 [15~20 ℃] → (C3H5O) 3P + 3HCl
Specific process can be classified into batch and continuous processes.
(1) Batch process
The phenol was added into the reactor, after warming to melt phosphorus trichloride was added to react with phenol at 70~90 ℃. After the phosphorus trichloride addition was completed, the temperature of reaction mixture was raised to about 150 ℃. After the removal of hydrogen chloride and unreacted phenol dissolved under reduced pressure at a high temperature, the product can be achieved.
(2) The use of a tower reactor
Phenol was feeding under the condenser located in the upper portion of the tower, while phosphorus trichloride enters above the receptacle located in the lower portion of the tower. Both reacted in the tower, and the product was collected in the receiver, meanwhile by-product hydrogen chloride was introduced into the absorber tower via the upper end of the condenser. After some process of the crude ester such as distillation, the product can be obtained.
Description
Triphenylphosphine: a member of tertiary phosphines
Triphenylphosphine (TPP) is a member of tertiary phosphines, which is phosphane, in which the three hydrogens are replaced by phenyl groups. It has a role as a reducing agent and an NMR chemical shift reference compound. It is a crucial ligand utilized in the Wittig reaction for alkene synthesis. This reaction involves the formation of alkyliden-etriphenylphosphoranes from the action of butyllithium or another base on the quarternary halide. Triphenylphosphine is used to synthesise organic compounds due to its nucleophilicity and reducing character.
Chemical Properties
Triphenylphosphine is a white to light tan flaked solid. Insoluble inwater; slightly soluble in alcohol; soluble in benzene, acetone, carbon tetrachloride. Combustible.
Uses
Triphenylphosphine is a versatile and efficient compound with a wide range of applications. It serves as a crucial ligand in homogeneous catalysts for petrochemical and fine chemical production, and as a co-catalyst in the production of isobutanol and n-butanol. It is also the basic raw material for rhodium phosphine complex catalysts, such as Wilkinson’s catalyst (RhCl(PPh3)3) for alkene hydrogenation and tetrakis(triphenylphosphine)palladium(0) for C-C coupling reactions in organic synthesis. In the dye industry, it is utilized as a sensitizer, heat stabilizer, light stabilizer, antioxidant, flame retardant, antistatic agent, rubber antiozonant, and analytical reagent. The rhodium and triphenylphosphine catalyst system is employed in the hydroformylation of vegetable oils and their methyl esters, and polymer-supported triphenylphosphine catalyzes the γ-addition of pronucleophiles to alkynoates. It also participates in the Heck reaction of 4-bromoanisole and ethyl acrylate in ionic liquids. Triphenylphosphine can be sulfonated to form trisulfonic acid and is used in Wittig synthesis as a standard ligand in homogeneous catalysis. It is involved in the synthesis of trimethyl phosphite, leading to the production of organophosphorus pesticides like dichlorvos, monocrotophos, and phosphamidon. Furthermore, it is used as a stabilizer in rubber and resin synthesis, an antioxidant in polyvinyl chloride, and a raw material in the synthesis of alkyd resins and polyester resins. Triphenylphosphine is also used in the synthesis of Chlorambucil, a cytotoxic agent for breast and pancreatic cancers, and in the preparation of α-Tocopherol analogues for monitoring antioxidant status.
Production Methods
Triphenylphosphine is one of the most widely used phosphorus-containing reagents in organic synthesis for many types of transformations such as the Mitsunobu, the Wittig, and the Staudinger reaction. Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium.
PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl
Synthesis Reference(s)
Tetrahedron Letters, 35, p. 625, 1994 DOI: 10.1016/S0040-4039(00)75855-2
Reactivity Profile
Triphenylphosphine reacts vigorously with oxidizing materials. .
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic; when heated to decomposition, emits highly toxic fumes of phosphine and POx.
Flammability and Explosibility
Not classified
reaction suitability
reagent type: reductant
Safety Profile
Moderately toxic by ingestion. Mildly toxic by inhalation. A skin and eye irritant. Combustible when exposed to heat or flame. Slight explosion hazard in the form of vapor when exposed to flame. Can react vigorously with oxidizing materials. To fight fire, use dry chemical, fog, CO2. When heated to decomposition it emits highly toxic fumes of phosphne and POx. See also PHOSPHINE and PHENOL.
Purification Methods
It crystallises from hexane, MeOH, diethyl ether, CH2Cl2/hexane or 95% EtOH. Dry it at 65o/<1mm over CaSO4 or P2O5. Chromatograph it through alumina using (4:1) *benzene/CHCl3 as eluent. [Blau & Espenson et al. J Am Chem Soc 108 1962 1986, Buchanan et al. J Am Chem Soc 108 1537 1986, Randolph & Wrighton J Am Chem Soc 108 3366 1986, Asali et al. J Am Chem Soc 109 5386 1987.] It has also been crystallised twice from pet ether and 5 times from Et2O/EtOH to give m 80.5o. Alternatively, dissolve it in conc HCl, and upon dilution with H2O it separates because it is weakly basic, it is then crystallised from EtOH/Et2O. It recrystallises unchanged from AcOH. [Forward et al. J Chem Soc Suppl. p121 1949, Muller et al. J Am Chem Soc 78 3557 1956.] 3Ph3P.4HCl crystallises out when HCl gas is bubbled through an Et2O solution, it has m 70-73o, but recrystallises very slowly and is deliquescent. The hydriodide, made by adding Ph3P to hydriodic acid, is not hygroscopic and decomposes at ~100o. The chlorate (1:1) salt has m 165-167o, but decomposes slowly at 100o. All salts hydrolyse in H2O to give Ph3P [IR, UV: Sheldon & Tyree J Am Chem Soc 80 2117 1958, pK: Henderson & Streuli J Am Chem Soc 82 5791 1960, Kosolapoff, Organophosphorus Compounds, Wiley 1950]. [Beilstein 16 IV 951.] § Available commercially on a polystyrene or polyethyleneglycol support.
FAQ
Q1: About the after-sale service of products
A: After purchasing the products from our factory, we have A professional technical team and after-sales team to serve you and solve all your problems in the future.
Q2: Can I get some samples?
A: Yes, we can provide samples, but the customer will pay the freight.
Q3: How do I start paying?
Payment can be made by wire transfer or T/T, apple_pay, google_pay, gc_real_time_bank_transfer , etc.
Q4: How to confirm product quality before placing an order?
A: You can get free samples of some products. You just have to pay the shipping fee or arrange for the sample to be sent to us by express.
You can send us your product specifications and requirements and we will produce products according to your requirements.
Q5: What is your MOQ?
A: The minimum quantity we can order is 1kg.
But usually we can accept a smaller quantity, say 100g, at the cost of 100% sample charge.
Q6: Shipping Time?
A: We ship the parcel out in 1-2 days and offer tracking No.. Shipping time is different to different country. Please consult
| ACF Chemical Co., Ltd.
Leon phone/whatsapp:008615950692266 email:md@acfchemical.com No. 45 Pengwan Road, Qianwan Bonded Port Area, Qingdao Area, China (Shandong) |
|
| DMEA | 108-01-0 |
| Dodecyl trimethyl ammonium chloride | 112-00-5 |
| N-Hexadecyltrimethylammonium chloride | 112-02-7 |
| 1831 | 112-03-8 |
| 1631Br | 57-09-0 |
| D821 | 5538-94-3 |
| D8/1021 | 68424-95-3 |
| D1021 | 7173-51-5 |
| D1821 | 61789-80-8 |
| TEP88 | 157905-74-3 |
| 1227 C12 | 139-07-1 |
| DMPT(N,N-Dimethyl-p-toluidine) | 99-97-8 |
| NDPT(N,N-dihydroxyethyl-p-toluidine) | 3077-12-1. |
| DMA(N,N-dimethylaniline) | 121-69-7 |
| N,N-Diethylaniline | 91-66-7 |
| MT(M-Toluidine) | 108-44-1 |
| PT(P-Toluidine) | 106-49-0 |
| O-Toluidine OT | 95-53-4 |
| Dimethyl(octyl)amine | 7378-99-6/1120-24-7 |
| C16-18-alkyldimethyl Octadecyl/Hexadecyl dimethylamines | 68390-97-6 |
| Octadecyl/behenyl dimethylamines | 124046-42-0 |
| N,N-dimethyldocosylamine | 21542-96-1 |
| N-Methyldioctylamine | 4455-26-9 |
| Di(octyl/decyl) methylamines | 308062-61-5 |
| Didecyl methylamine | 7396-58-9 |
| N-methyldidodecylamine | 2915-90-4 |
| Dipalmitamine | 16724-61-1 |
| Trioctylamine | 1116-76-3 |
| Trioctylamine | 68814-95-9 |
| N-3-Laurylamidopropyl dimethylamine | 3179-80-4 |
| N-3-(Hydrogenated cocoamido)propyl dimethylamines | 288095-05-6 |
| N-3-Oleylamidopropyl dimethylamine | 109-28-4 |
| N-3-Erucylamidopropyl dimethylamine | 60270-33-9 |
| N-Oleyl 1,3-propanediamine | 7173-62-8 |
| Bis(aminopropyl)laurylamine | 2372-82-9 |
| N-tallow alkyltripropylenetetra | 68911-79-5 |
| 3-(isodecyloxy)propylamine | 30113-45-2 |
| N-[3-(isodecyloxy)propyl]propane-1,3-diamine | 72162-46-0 |
| 2-(Methylamino)ethanol | 109-83-1 |
| N-Methyldiethanolamine | 105-59-9 |
| 3-Methoxy propyl amine | 5332-73-0 |
| N,N-dimethylcyclohexylamine | 98-94-2 |
| 1,3,5-Tris[3-(dimethylamino)propyl]hexahydro-1,3,5-triazine | 15875-13-5 |
| N,N,N’-trimethylamino-N’-ethylethanolamine | 2212-32-0 |
| N,N-Dimethylethanolamine | 108-01-1 |
| Acetone | |
| Acrylic acid | |
| Adipic acid | |
| Alpha-Methylstyrene (AMS) | |
| Benzoic Acid | |
| Bisphenol A | |
| Butyl Acrylat (BA) | |
| Butyl acetate (Butac) | |
| Butyl diglycol (BDG) | |
| Butyl glycol | |
| Para-tertiary butyl benzoic acid (PTBBA) | |
| n-Butanol | |
| n-Butyl methacrylate (n-BUMA) | |
| para-tert. Butylphenol (PTBP) | |