product description
Basic Info.
Nitrilotriacetic acid Properties
| Melting point | 245 °C (dec.)(lit.) |
|---|---|
| Boiling point | 326.86°C (rough estimate) |
| Density | 1.5784 (rough estimate) |
| vapor pressure | 0-0Pa at 20-25℃ |
| refractive index | 1.4860 (estimate) |
| Flash point | 100 °C |
| storage temp. | 2-8°C |
| solubility | 0.1 M NaOH: 0.1 M at 20 °C, clear, colorless |
| form | Powder |
| pka | 3.03, 3.07, 10.70(at 20℃) |
| color | White |
| PH | 1.7-2.7 (23°C, 10g/L) |
| Water Solubility | Insoluble. <0.01 g/100 mL at 23 ºC |
| λmax | λ: 260 nm Amax: 1.5 λ: 280 nm Amax: 0.05 |
| Merck | 14,6579 |
| BRN | 1710776 |
| InChI | 1S/C6H9NO6/c8-4(9)1-7(2-5(10)11)3-6(12)13/h1-3H2,(H,8,9)(H,10,11)(H,12,13) |
| InChIKey | MGFYIUFZLHCRTH-UHFFFAOYSA-N |
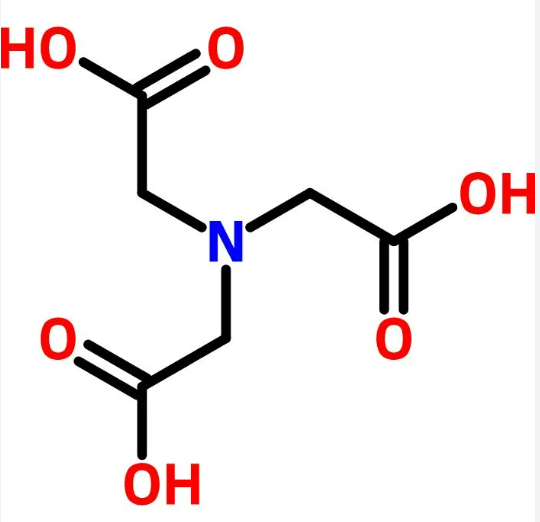
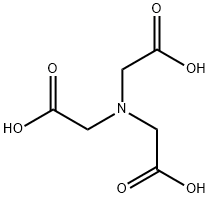
| SMILES | OC(=O)CN(CC(O)=O)CC(O)=O |
| LogP | -3.81 at 25℃ |
| Toxicology and Carcinogenesis | Bioassays of Nitrilotriacetic Acid (NTA) and Nitrilotriacetic Acid, Trisodium Salt, Monohydrate (Na3-NTA-H2O) for Possible Carcinogenicity (CASRN 139-13-9) (NTA) (CASRN 18662-53-8) (Na3-NTA-H2O) |
| CAS DataBase Reference | 139-13-9(CAS DataBase Reference) |
| FDA UNII | KA90006V9D |
| IARC | 2B (Vol. 48, 73) 1999 |
| Proposition 65 List | Nitrilotriacetic Acid |
| NIST Chemistry Reference | Nitrilotriacetic acid(139-13-9) |
| EPA Substance Registry System | Nitrilotriacetic acid (139-13-9) |
| UNSPSC Code | 41116107 |
| NACRES | NA.31 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H351 |
| Precautionary statements | P201-P305+P351+P338-P308+P313 |
| PPE | Eyeshields, Gloves, type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn,T |
| Risk Statements | 22-36-40-36/37/38-45-20/21/22 |
| Safety Statements | 26-36/37-45-36/37/39-53 |
| RIDADR | 2811 |
| WGK Germany | 2 |
| RTECS | AJ0175000 |
| F | 21 |
| TSCA | TSCA listed |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 29224995 |
| Storage Class | 11 – Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Oral Carc. 2 Eye Irrit. 2 |
| Hazardous Substances Data | 139-13-9(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 1100 mg/kg |
Nitrilotriacetic acid Chemical Properties,Uses,Production
Chemical Properties
Nitrilotriacetic acid, also known as NTA, is a white crystalline powder. It can dissolve in ammonia and alkali solutions while being slightly soluble in hot water. However, it is insoluble in water and most organic solvents. NTA can form mono-, di-, and tribasic salts, all of which are soluble in water. Combustible. 70% biodegradable.
Uses
Used in sequestration of metals; chelometric analysis.
Uses
Nitrilotriacetic acid is a chelating agent which forms coordination compounds with metal ions. Nitrilotriacetic acid is used in complexometric titrations and as well as for protein isolation and purification in the His-tag method. This compound is a contaminant of emerging concern (CECs).
Definition
ChEBI: Nitrilotriacetic acid is a tricarboxylic acid and a NTA. It has a role as a nephrotoxic agent and a carcinogenic agent. It is a conjugate acid of a nitrilotriacetate(1-).
Preparation
The synthesis of nitrilotriacetic acid involves the following steps:
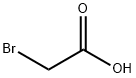
Chloroacetic acid is reacted with sodium hydroxide to produce sodium chloroacetate.
Sodium chloroacetate then reacts with ammonium chloride to form sodium aminotriacetate.
The resulting sodium aminotriacetate is acidified to obtain nitrilotriacetic acid.
Synthesis Reference(s)
The Journal of Organic Chemistry, 15, p. 46, 1950 DOI: 10.1021/jo01147a008
Air & Water Reactions
Water Insoluble.
Reactivity Profile
Nitrilotriacetic acid is incompatible with strong oxidizers, aluminum, copper, copper alloy and nickel. Nitrilotriacetic acid is also incompatible with strong bases.
Hazard
Possible carcinogen.
Health Hazard
Toxicity and health hazard of Nitrilotriacetic acid is low. Contact with eyes causes irritation.
Fire Hazard
Flash point data for Nitrilotriacetic acid are not available; however, Nitrilotriacetic acid is probably combustible.
Flammability and Explosibility
Non flammable
reaction suitability
reagent type: reductant
Biological Activity
Nitrilotriacetic (NTA) has the ability to link with histidine side chain of proteins. It can be used in fluorescent labelling, hexahistidine (His6)-tagged proteins for purification and surface immobilization.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx,.
Potential Exposure
Nitrilotriacetic acid (NTA) was used as a phosphate replacement in laundry detergents in the late 1960s. In 1971, the use of NTA was discontinued. The possibility of resumed use arose in 1980. NTA is now used in laundry detergents in states where phosphates are banned. NTA is also used as a boiler feed-water additive at a maximum use level of 5 ppm of trisodium salt. Currently, the remaining nondetergent uses of NTA are for water treatment, textile treatment; metal plating and cleaning; and pulp and paper processing.
Carcinogenicity
Nitrilotriacetic acid is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Nitrilotriacetic acid Preparation Products And Raw materials
FAQ
Q1: About the after-sale service of products
A: After purchasing the products from our factory, we have A professional technical team and after-sales team to serve you and solve all your problems in the future.
Q2: Can I get some samples?
A: Yes, we can provide samples, but the customer will pay the freight.
Q3: How do I start paying?
Payment can be made by wire transfer or T/T, apple_pay, google_pay, gc_real_time_bank_transfer , etc.
Q4: How to confirm product quality before placing an order?
A: You can get free samples of some products. You just have to pay the shipping fee or arrange for the sample to be sent to us by express.
You can send us your product specifications and requirements and we will produce products according to your requirements.
Q5: What is your MOQ?
A: The minimum quantity we can order is 1kg.
But usually we can accept a smaller quantity, say 100g, at the cost of 100% sample charge.
Q6: Shipping Time?
A: We ship the parcel out in 1-2 days and offer tracking No.. Shipping time is different to different country. Please consult
| ACF Chemical Co., Ltd.
Leon phone/whatsapp:008615950692266 email:md@acfchemical.com No. 45 Pengwan Road, Qianwan Bonded Port Area, Qingdao Area, China (Shandong) |
|
| DMEA | 108-01-0 |
| Dodecyl trimethyl ammonium chloride | 112-00-5 |
| N-Hexadecyltrimethylammonium chloride | 112-02-7 |
| 1831 | 112-03-8 |
| 1631Br | 57-09-0 |
| D821 | 5538-94-3 |
| D8/1021 | 68424-95-3 |
| D1021 | 7173-51-5 |
| D1821 | 61789-80-8 |
| TEP88 | 157905-74-3 |
| 1227 C12 | 139-07-1 |
| DMPT(N,N-Dimethyl-p-toluidine) | 99-97-8 |
| NDPT(N,N-dihydroxyethyl-p-toluidine) | 3077-12-1. |
| DMA(N,N-dimethylaniline) | 121-69-7 |
| N,N-Diethylaniline | 91-66-7 |
| MT(M-Toluidine) | 108-44-1 |
| PT(P-Toluidine) | 106-49-0 |
| O-Toluidine OT | 95-53-4 |
| Dimethyl(octyl)amine | 7378-99-6/1120-24-7 |
| C16-18-alkyldimethyl Octadecyl/Hexadecyl dimethylamines | 68390-97-6 |
| Octadecyl/behenyl dimethylamines | 124046-42-0 |
| N,N-dimethyldocosylamine | 21542-96-1 |
| N-Methyldioctylamine | 4455-26-9 |
| Di(octyl/decyl) methylamines | 308062-61-5 |
| Didecyl methylamine | 7396-58-9 |
| N-methyldidodecylamine | 2915-90-4 |
| Dipalmitamine | 16724-61-1 |
| Trioctylamine | 1116-76-3 |
| Trioctylamine | 68814-95-9 |
| N-3-Laurylamidopropyl dimethylamine | 3179-80-4 |
| N-3-(Hydrogenated cocoamido)propyl dimethylamines | 288095-05-6 |
| N-3-Oleylamidopropyl dimethylamine | 109-28-4 |
| N-3-Erucylamidopropyl dimethylamine | 60270-33-9 |
| N-Oleyl 1,3-propanediamine | 7173-62-8 |
| Bis(aminopropyl)laurylamine | 2372-82-9 |
| N-tallow alkyltripropylenetetra | 68911-79-5 |
| 3-(isodecyloxy)propylamine | 30113-45-2 |
| N-[3-(isodecyloxy)propyl]propane-1,3-diamine | 72162-46-0 |
| 2-(Methylamino)ethanol | 109-83-1 |
| N-Methyldiethanolamine | 105-59-9 |
| 3-Methoxy propyl amine | 5332-73-0 |
| N,N-dimethylcyclohexylamine | 98-94-2 |
| 1,3,5-Tris[3-(dimethylamino)propyl]hexahydro-1,3,5-triazine | 15875-13-5 |
| N,N,N’-trimethylamino-N’-ethylethanolamine | 2212-32-0 |
| N,N-Dimethylethanolamine | 108-01-1 |
| Acetone | |
| Acrylic acid | |
| Adipic acid | |
| Alpha-Methylstyrene (AMS) | |
| Benzoic Acid | |
| Bisphenol A | |
| Butyl Acrylat (BA) | |
| Butyl acetate (Butac) | |
| Butyl diglycol (BDG) | |
| Butyl glycol | |
| Para-tertiary butyl benzoic acid (PTBBA) | |
| n-Butanol | |
| n-Butyl methacrylate (n-BUMA) | |
| para-tert. Butylphenol (PTBP) | |